Meldung vom:
New publications on the thermodynamic properties of Fe oxides, sulfates and arsenates
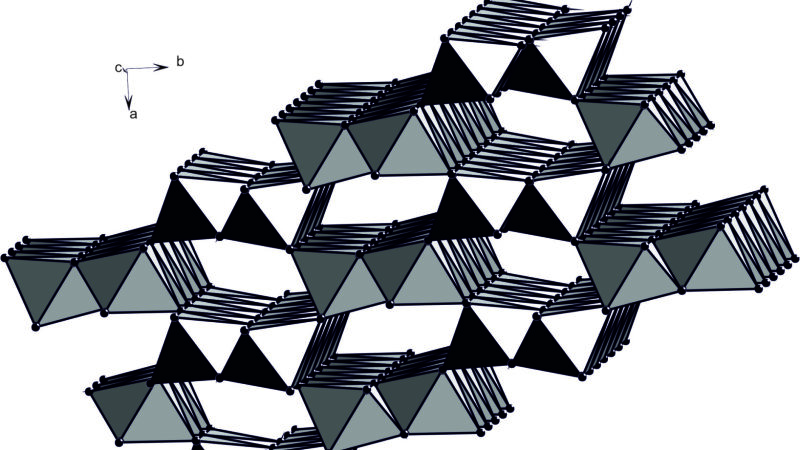
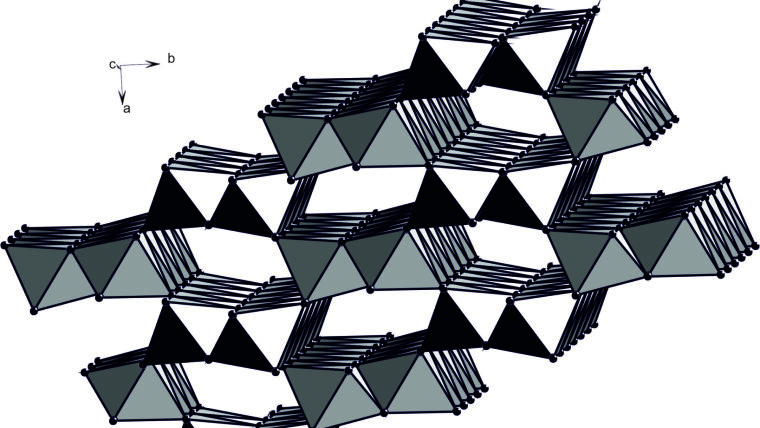
Some of the technologically important metals are also rare in the nature and considered as critical from the point of their accessibility and resources. In our work, we were interested in thermodynamic properties of Fe oxides, sulfates, and arsenates that take up some of the critical metals (indium, gallium, scandium) in their structures. Using these thermodynamic properties, extraction of the metals from natural materials (lateritic profiles) or waste products (red mud from processing of bauxite) could be optimized. We found that in all systems studied, critical metals strongly partition into the aqueous solution whereas Fe prefers the solid phase. The thermodynamic prediction can be imprecise if adsorption of the metals onto the surfaces of the Fe oxides prevails, as suggested by some field studies. More information about our thermodynamic work can be found in two new publications at https://doi.org/10.1016/j.jct.2024.107308Externer Link and https://doi.org/10.1007/s00269-024-01298-1Externer Link.
Crystal structure of goethite
Foto: Prof. Dr. Juraj Majzlan