Preprints
-
3. M. Ringled, A. Eith, S. Zechel, U. S. Schubert, K. Jablonka, J. Schneidewind, ChemRxiv 2025; Kinetically guided exploration of photocatalytic reactions by combining automation with in situ measurements.
Mehr erfahrenExterner LinkenPhotocatalysis enables valuable reactions such as synthetic transformations or energy conversion processes like water splitting. To rationally improve photocatalytic reactions, mechanistic insights are required. These can be obtained with kinetic measurements, which are, however, difficult to obtain for a large enough number of reaction conditions to provide systematic and valuable insights. To this end, we present a system for performing photocatalytic reactions that combines time-resolved in situ measurements with a fully automated process for liquid handling, light source control, dynamic feedback and automated kinetic data evaluation, enabled by an open-source Python framework. The system is applied to study photocatalytic water oxidation using [Ru(bpy)3]2+ as both photosensitizer and water oxidation catalyst, with oxygen formation being measured in situ. Using this benchmark reaction, we investigate the effect of different reaction parameters (catalyst and sacrificial oxidant concentration, irradiance, pH-value) on the rate of oxygen evolution. The results show that the automated process enables highly reproducible experiments while obtaining full time-concentration curves with a high temporal resolution (seconds) for each experiment. Using the obtained data we derive a chemical reaction network and rate constants to describe the detailed mechanism of the photocatalytic reaction. This kinetic model reveals an unexpected light-driven step to convert [Ru(bpy)3]3+ and water to hydrogen peroxide (which ultimately disproportionates to form oxygen) as well as two competing deactivation pathways, unimolecular and bimolecular decomposition, the latter of which is autocatalytic. These results demonstrate how the combination of in situ kinetic measurements and automation unlocks the data- driven exploration of the chemical space, producing novel mechanistic insights.
-
2. W, Tuschewitzki, J. Lange, J. Schneidewind, M. Kaltschmitt, arXiv 2025; Impacts of photocatalytic hydrogen production on the European energy system.
Mehr erfahrenExterner LinkenEspecially in regions with high solar irradiation, photocatalysis presents a promising low-cost "green" hydrogen production option. Thus, this paper analyzes impacts of increasing photocatalysis shares on the European energy system using an open-source energy system optimization model covering the electricity, industry, and heating sectors with high spatial and temporal resolution. Photocatalysis deployment is investigated at various market shares by exogenously altering photocatalysis costs. The results show that integrating photocatalysis necessitates systematic adjustments since it lacks the flexible load attributes of water electrolysis. Therefore, a significant geographic shift in hydrogen production and demand from the Northwest to South Europe is expected in the case of large-scale photocatalysis adoption. Despite these challenges, installed photocatalysis shows costs within the photocatalysis cost projections. Thus, photocatalysis could contribute to a critical diversification of hydrogen production, easing material demands for other renewable technologies. Nevertheless, it requires strategic planning to avoid lock-ins and to maximize its potential.
-
1. N. Brezhneva, J. Schneidewind, ChemRxiv 2025; Testing of particulate photocatalysts for water splitting.
Mehr erfahrenExterner LinkenLight-driven water splitting using particulate photocatalysts could enable low-cost production of solar fuels, if sufficiently active, stable and efficient catalysts are developed. However, to enable continuous progress in this direction, it is crucial that photocatalysts are tested using reproducible and insightful experimental protocols. Herein, we describe a simple protocol that also works with small reaction volumes, allowing for explorative testing of photocatalysts that are only available in limited amounts. At the same time, all crucial experimental parameters (e.g. reaction temperature, irradiation intensity) are precisely measured and controlled and reaction products, such as oxygen, can be measured in situ. The protocol covers preparation of the photocatalyst dispersion, details regarding the experimental set- up, the procedure for irradiation experiments, measurement of product formation (for oxygen, hydrogen and hydrogen peroxide) and corresponding data analysis.
Publikationen
-
11. N. Belko, H. Maltanava, N. Brezhneva, K. Tamarov, V.‐P. Lehto, J. O Moilanen, J. TT Leskinen, D. Semenov, E. Filonenko, I. Koshevoy, J. Schneidewind, W. H De Vos, P. Kuzhir, Global Challenges 2026, 10(1), e00447; Engineering Carbon Nitride Quantum Dots via Sulfur Doping for Controlled Reactive Oxygen Species Generation.
Mehr erfahrenExterner LinkenCarbon nitride quantum dots (CNQDs) are emerging as versatile photocatalytic materials with promising applications in biomedicine and environmental remediation. In this study, we synthesized pristine and sulfur‐doped CNQDs via a hydrothermal method, and characterized them using transmission electron microscopy (TEM), X‐ray photoelectron spectroscopy (XPS), and UV–vis absorption spectroscopy. For the first time, the quantum yields of superoxide and singlet oxygen generation were measured for CNQDs. Sulfur doping was found to significantly enhance superoxide generation while concurrently suppressing singlet oxygen production, offering a powerful mechanism for tailoring reactive oxygen species (ROS) output. In addition, all CNQD samples produced hydrogen peroxide and hydroxyl radicals. The ability of these nanomaterials to produce multiple ROS types underscores their potential as hypoxia‐resistant photosensitizers (PSs) for photodynamic therapy (PDT) and as efficient photocatalysts for pollutant degradation.
-
10. Sambur et al., ACS Energy Letters 2025; Gerischer Elechtrochemistry Today.
Semiconductor photoelectrochemistry is a dynamic and interdisciplinary field at the forefront of research in solar fuels, energy conversion, and catalysis. This Perspective captures the collective insights from the 2nd Gerischer Electrochemistry Today Symposium, held at Colorado State University in Fort Collins, CO, in August 2025, which convened leading researchers, early-career scientists, and industry partners to define the critical next steps for the field. Through interactive sessions, technical talks, panel discussions, and training initiatives—including a Semiconductor Electrochemistry Bootcamp—the symposium emphasized three pillars of advancement: (i) facilitating the exchange of new ideas in semiconductor electrochemistry and charge separation; (ii) fostering the development of future researchers, research topics and participation in the semiconductor workforce; and (iii) building community. This Energy Focus article distills key themes from the meeting and identifies major knowledge gaps in the following areas: mechanisms of charge separation and recombination, role of defects and disorder, dynamic and operando characterization methods, interfacial chemistry and surface passivation, theoretical and modeling limitations, and standardization and benchmarking. The inclusive and collaborative structure of the symposium enabled the generation of this comprehensive report that will serve as a roadmap for fundamental and applied research in the rapidly evolving field of semiconductor electrochemistry over the next decade.
-
9. L. Ni, E. Troschke, H. Vorwerk, S. S. Avarvand, A. Stihl, N. Brezhneva, M. Hermesdorf, D. Leistenschneider, F. H. Schacher, J. Schneidewind, M. Oschatz, Advanced Functional Materials 2025, e04107; Engineering Porous Hollow Metal-Poly(Heptazine Imide) Spheres: An Optimized Synthetic Strategy for Controlling Surface, Morphology, and Properties.
Mehr erfahrenExterner LinkenThe utilization of carbon nitride materials is significantly constrained by their inherently low specific surface area (SA). Specifically, introducing porosity into their ionic derivatives, such as poly(heptazine imide) (PHI), proves challenging as the high-temperature ionothermal synthesis produces more regular and stacked structures. This study introduces a novel synthetic strategy for porous hollow PHI spheres (HS_MPHIs) via a hard-templating method. HS_MPHIs exhibit significantly higher SA (145 m2 g−1 vs 44 m2g−1 of bulk KPHI), precise metal content control, improved visible light absorption, adjustable band positions, and tunable hydrophilicity. The increased SA facilitates the intercalation of a higher content of metal cations, with PHI channel system accommodating various cations, including potassium, iron, nickel, and cobalt. Transition metal containing PHIs show notable morphological, structural and optical changes, such as flower-like shapes, varied interlayer distances and SA, extended light absorption to 700 nm, and more negative valence band positions. The optimized HS_NiPHI_3_0.4 achieves a 1.4-fold higher H2O2 production than the bulk material via a 2e‒ O2 reduction pathway under scavenger-free conditions, with relatively stable performance over four cycles.
-
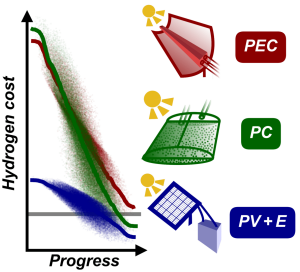
8. J. Schneidewind*, Advanced Energy Materials 2022, 12, 2200342; How much technological progress is needed to make solar hydrogen cost-competitive?
Mehr erfahrenExterner LinkTable of Contents Advanced Energy Materials 2022
Grafik: Jacob SchneidewindCost-effective production of green hydrogen is a major challenge for global adoption of a hydrogen economy. Technologies such as photoelectrochemical (PEC) or photocatalytic (PC) water splitting and photovoltaic + electrolysis (PV+E) allow for sustainable hydrogen production from sunlight and water, but are not yet competitive with fossil fuel-derived hydrogen. Herein, open-source software for techno-economic analysis (pyH2A) along with a Monte Carlo- based methodology for modelling of technological progress are developed. Together, these tools allow for the study of required technological improvement to reach a competitive target cost. They are applied to PEC, PC, and PV+E to identify required progress for each and derive actionable research targets. For PEC, it is found that cell lifetime improvements (>2 years) and operation under high solar concentration (>50-fold) are crucial, necessitating systems with high space-time yields. In the case of PC, solar-to-hydrogen efficiency has to reach at least 6%, and lowering catalyst concentration (<0.2 g L−1) by improving absorption properties is identified as a promising path to low-cost hydrogen. PV+E requires ≈two or threefold capital cost reductions for photovoltaic and electrolyzer components. It is hoped that these insights can inform materials research efforts to improve these technologies in the most impactful ways.
-
7. A. Hamza*, D. Moock, C. Schlepphorst, J. Schneidewind, W. Baumann, F. Glorius*, Chemical Science 2022, 13, 985-995; Unveiling a key catalytic pocket for the ruthenium NHC- catalyzed asymmetric heteroarene hydrogenation.
Mehr erfahrenExterner LinkThe chiral ruthenium(II)bis-SINpEt complex is a versatile and powerful catalyst for the hydrogenation of a broad range of heteroarenes. This study aims to provide understanding of the active form of this privileged catalyst as well as the reaction mechanism, and to identify the factors which control enantioselectivity. To this end we used computational methods and in situ NMR spectroscopy to study the hydrogenation of 2-methylbenzofuran promoted by this system. The high flexibility and conformational freedom of the carbene ligands in this complex lead to the formation of a chiral pocket interacting with the substrate in a “lock-and-key” fashion. The non-covalent stabilization of the substrate in this particular pocket is an exclusive feature of the major enantiomeric pathway and is preserved throughout the mechanism. Substrate coordination leading to the minor enantiomer inside this pocket is inhibited by steric repulsion. Rather, the catalyst exhibits a “flat” interaction surface with the substrate in the minor enantiomer pathway. We probe this concept by computing transition states of the rate determining step of this reaction for a series of different substrates. Our findings open up a new approach for the rational design of chiral catalysts.
-
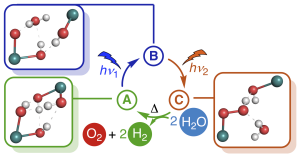
6. J. Schneidewind*, M. A. Argüello-Cordero, H. Junge, S. Lochbrunner, M. Beller, Energy & Environmental Science 2021, 14, 4427-4436; Two-photon, visible light water splitting at a molecular ruthenium complex.
Mehr erfahrenExterner LinkÜbersicht Zwei-Photonen Wasserspaltung
Grafik: Jacob SchneidewindWater splitting to give molecular oxygen and hydrogen or the corresponding protons and electrons is a fundamental four-electron redox process, which forms the basis of photosynthesis and is a promising approach to convert solar into chemical energy. Artificial water splitting systems have struggled with orchestrating the kinetically complex absorption of four photons as well as the difficult utilization of visible light. Based on a detailed kinetic, spectroscopic and computational study of Milstein’s ruthenium complex, we report a new mechanistic paradigm for water splitting, which requires only two photons and offers a new method to extend the range of usable wavelengths far into the visible region. We show that two-photon water splitting is enabled by absorption of the first, shorter wavelength photon, which produces an intermediate capable of absorbing the second, longer wavelength photon (up to 630 nm). The second absorption then causes O–O bond formation and liberation of O2. Theoretical modelling shows that two-photon water splitting can be used to achieve a maximum solar-to-hydrogen efficiency of 18.8%, which could be increased further to 28.6% through photochemical instead of thermal H2 release. It is therefore possible to exceed the maximum efficiency of dual absorber systems while only requiring a single catalyst. Due to the lower kinetic complexity, intrinsic utilization of a wide wavelength range and high-performance potential, we believe that this mechanism will inspire the development of a new class of water splitting systems that go beyond the reaction blueprint of photosynthesis.
-
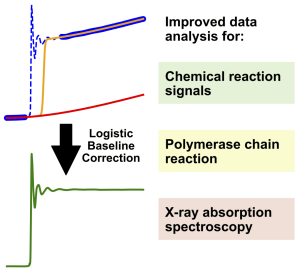
5. J. Schneidewind*, Hrishi Olickel, Chemistry – Methods 2021, 1, 2, 89 -100; Improving Data Analysis in Chemistry and Biology Through Versatile Baseline Correction.
Mehr erfahrenExterner LinkTable of Contents Chemistry Methods 2020
Grafik: Jacob SchneidewindAccurate data analysis is a cornerstone for the meaningful interpretation of measurements in chemistry and biology. To enable accurate analysis, it is often necessary to remove the background from a measurement via baseline correction, as is commonly done for spectroscopy or chromatography. However, no equivalent methods for baseline correction exist for an entire group of measurements, which includes chemical reaction measurements, quantitative polymerase chain reaction and X-ray absorption spectroscopy. This is because these measurements give rise to a different class of features in their signals, which prevent the application of classical baseline correction methods. In this work, a general method for baseline correction of these features is developed, which is shown to simplify and improve data analysis for these measurements. Through publicly accessible and easy to use software we expect this method to be broadly useful to improve and simplify data analysis for chemists and biologists.
-
4. D. Moock, M. P . Wiesenfeldt, M. Freitag, S. Muratsugu, S. Ikemoto, R. Knitsch, J. Schneidewind, W. Baumann, A. H. Schäfer, A. Timmer, M. Tada, M. R. Hansen, F. Glorius*, ACS Catalysis 2020, 10, 11, 6309-6317; Mechanistic Understanding of the Heterogeneous, Rhodium-Cyclic(Alkyl)(Amino)Carbene-Catalyzed (Fluoro-)Arene Hydrogenation.
Mehr erfahrenExterner LinkRecently, chemoselective methods for the hydrogenation of fluorinated, silylated, and borylated arenes have been developed, providing direct access to previously unattainable, valuable products. Herein, a comprehensive study on the employed rhodium-cyclic (alkyl)(amino)carbene (CAAC) catalyst precursor is disclosed. Mechanistic experiments, kinetic studies, and surface-spectroscopic methods revealed supported rhodium(0) nanoparticles (NP) as the active catalytic species. Further studies suggest that CAAC-derived modifiers play a key role in determining the chemoselectivity of the hydrogenation of fluorinated arenes, thus offering an avenue for further tuning of the catalytic properties.
-
3. S. Kreft, R. Schoch, J. Schneidewind, J. Rabeah, E. V. Kondratenko, V. A. Kondratenko, H. Junge, M. Bauer, S. Wohlrab, M. Beller*, Chem 2019, 5, 1818–1833; Improving Selectivity and Activity of CO2 Reduction Photocatalysts with Oxygen.
Mehr erfahrenExterner LinkA highly porous photocatalyst (copper on TiO2 aerogel) was synthesized and applied in aqueous CO2 reduction without using external sacrificial electron donors. For the first time, complete selectivity toward CO and improved catalyst productivity are observed in the presence of oxygen. The optimal activity is achieved in a feed containing 0.5 vol% O2 in CO2. In situ XAS, EPR, and UV-vis measurements suggest, among different Cu oxidation states, Cu(I) to be the most active species in photocatalytic CO2 reduction. Oxygen sensing of the catalyst in the presence of O2/CO2 mixtures indicated an unexpected photoadsorption of oxygen on the titania surface. We propose photooxidation of surface hydroxyl groups to be the electron source for CO2 reduction, which is supported by hydroxyl group consumption, detection of hydroxyl radicals using in situ EPR, and detection of surface peroxide species after the reaction.
-
2. T. Senthamarai, K. Murugesan, J. Schneidewind, N. V. Kalevaru, W. Baumann, H. Neumann, P. C. J. Kamer, M. Beller*, R. V. Jagadeesh*, Nature Communications 2018, 9, 1– 12; Simple ruthenium-catalyzed reductive amination enables the synthesis of a broad range of primary amines.
Mehr erfahrenExterner LinkThe production of primary benzylic and aliphatic amines, which represent essential feed- stocks and key intermediates for valuable chemicals, life science molecules and materials, is of central importance. Here, we report the synthesis of this class of amines starting from carbonyl compounds and ammonia by Ru-catalyzed reductive amination using H2. Key to success for this synthesis is the use of a simple RuCl2(PPh3)3 catalyst that empowers the synthesis of >90 various linear and branched benzylic, heterocyclic, and aliphatic amines under industrially viable and scalable conditions. Applying this catalyst, −NH2 moiety has been introduced in functionalized and structurally diverse compounds, steroid derivatives and pharmaceuticals. Noteworthy, the synthetic utility of this Ru-catalyzed amination protocol has been demonstrated by upscaling the reactions up to 10 gram-scale syntheses. Further- more, in situ NMR studies were performed for the identification of active catalytic species. Based on these studies a mechanism for Ru-catalyzed reductive amination is proposed.
-
1. J. Schneidewind, R. Adam, W. Baumann, R. Jackstell, M. Beller*, Angewandte Chemie Int. Ed. 2017, 56, 1890–1893; Low-Temperature Hydrogenation of Carbon Dioxide to Methanol with a Homogeneous Cobalt catalyst.
Mehr erfahrenExterner LinkHerein we describe the first homogeneous non-noble metal catalyst for the hydrogenation of CO2 to methanol. The catalyst is formed in situ from [Co(acac)3], Triphos, and HNTf2 and enables the reaction to be performed at 100 °C without a decrease in activity. Kinetic studies suggest an inner-sphere mechanism, and in situ NMR and MS experiments reveal the formation of the active catalyst through slow removal of the acetylacetonate ligands.
Andere publikationen (nicht peer-reviewed)
-
2. M. W. Tausch, J. Schneidewind*, Chemie in userer Zeit 2023, DOI: 10.1002/ciuz.202300016; Mit Licht zu grünem Wasserstoff.
Mehr erfahrenExterner LinkGrüner Wasserstoff ist ein Hauptakteur in der Energiewende. Während im öffentlichen Diskurs der Fokus auf grünem Wasserstoff liegt, der via Photovoltaik oder Windenergie und Wasserelektrolyse produziert wird, informiert dieser Beitrag über den aktuellen Stand bei der Erforschung der direkten Wasserspaltung mit Licht. Dabei wird eingegangen auf die grundlegenden Konzepte und Prozesse in der Wasserspaltung, bisherige Umsetzungen sowie aktuelle Herausforderungen, um diese Technologie in die Anwendung zu bringen. Um die noch anstehenden Herausforderungen zu überwinden ist auch die Einbindung dieser Thematik in den Chemieunterricht notwendig. Es wird auf Experimente, digitale Medien, und Lehrkonzepte, die dafür entwickelt wurden, hingewiesen.
-
1. J.Schneidewind*, Nachrichten aus der Chemie 2022, 70, 66-67; Grüner Wasserstoff direkt aus Sonnenlicht.
Mehr erfahrenExterner LinkGibt es Wege, Wasser mit Licht zu spalten, die nicht die natürliche Photosynthese zum Vorbild haben? Jacob Schneidewind ist ihnen auf der Spur: Er entwickelt Photokatalysatoren dafür.