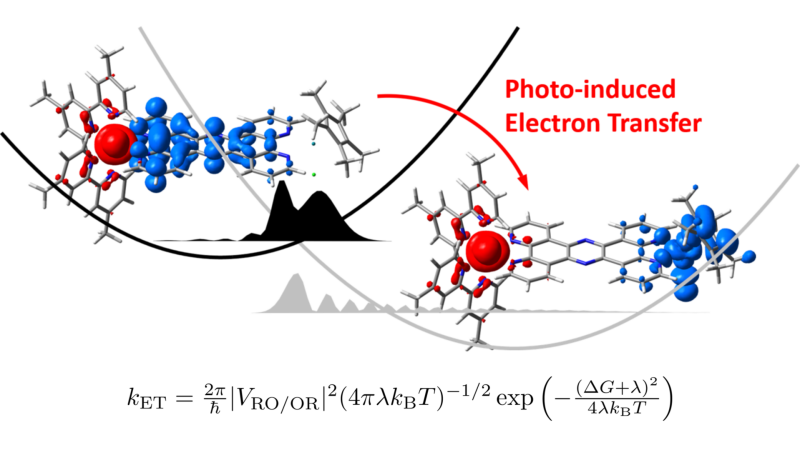
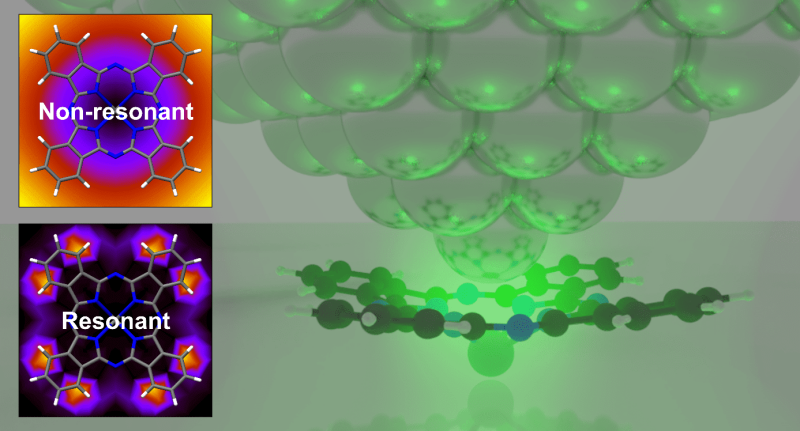
Structural Control of Metal-Centered Excited States in Cobalt(III) Complexes via Bite Angle and π–π Interactions
Year of publicationPublished in:Journal of the American Chemical Society
- University Bibliography Jena:
- fsu_mods_00027038External link